De-Risking Behavioral Health Environments
Working collaboratively with some of the world’s leading behavioral health organizations, Kingsway continuously innovate at the forefront of ligature-resistant product design and manufacture, de-risking for our clients at every stage.
Our approach takes a holistic view, working step-by-step to reduce risk for our clients across each of these areas. This comprehensive program is designed to deliver peace of mind in high risk and unpredictable environments.

Potential risk exists in the following areas…
Patient Self-Harm
- The risk of patient self-harm or loss of life and the potential for litigation
Staff Injury
- The risk of harm to staff and the potential for litigation around violent incidents or injury
Non-Compliance
- Accreditation issues due to poor ligature mitigation and risk control
Slow Patient Recovery
- Poorly designed environment impacts patient recovery times and damages hospital reputation
Under (or over) Specification of Product
- Unsuitable products leading to financial loss and health & safety issues
Incorrect Assembly of Product
- Leading to safety concerns, damage, litigation, and financial loss
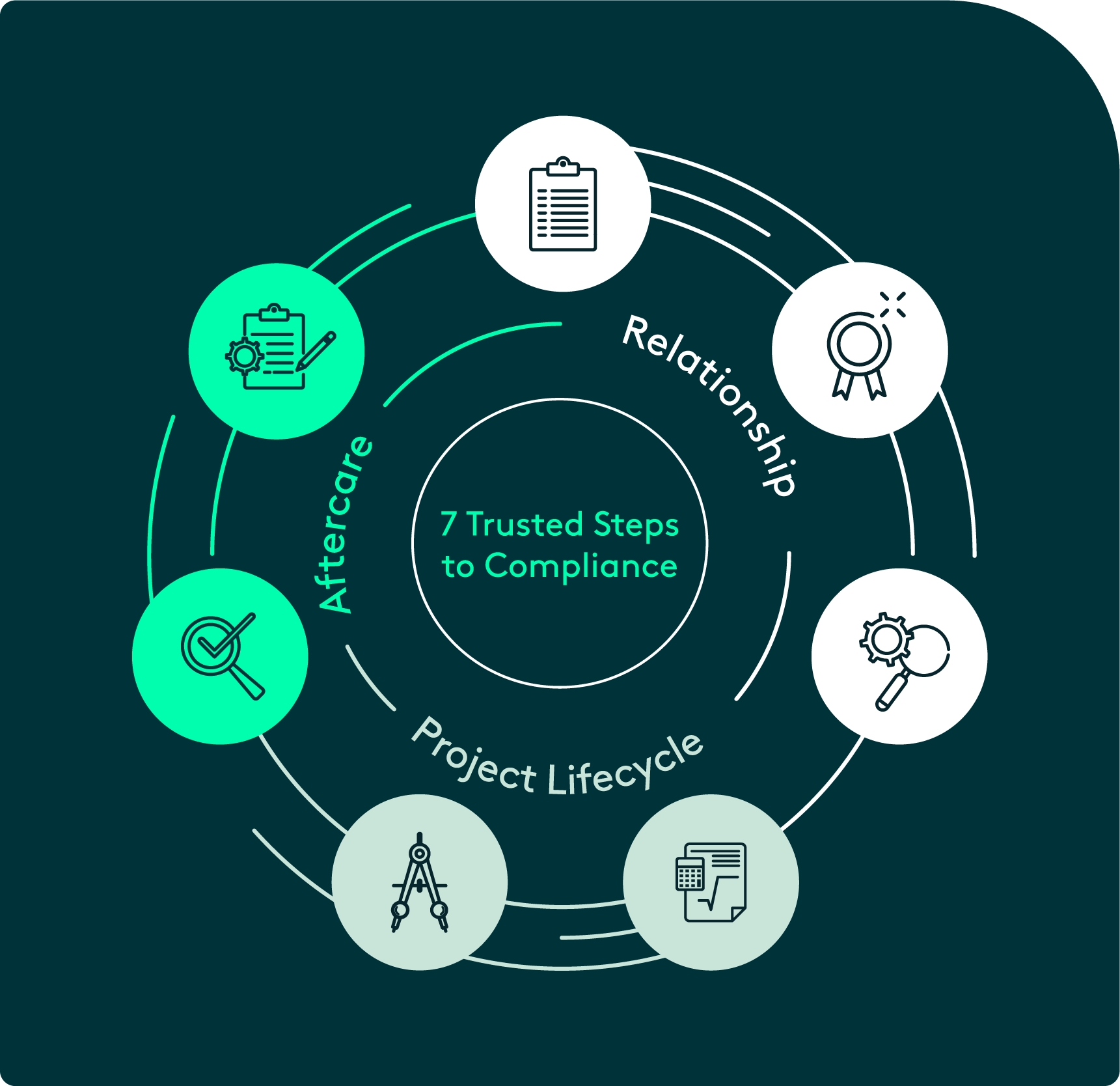
The Kingsway approach:
7 trusted steps to compliance
The human, financial and environmental impact of ineffective ligature-resistant products and strategies can be considerable.
Kingsway’s 7-Step approach is designed to reduce suicide risks around doors and bathrooms in behavioral health environments.
The national cost of suicides and self injury in the United States in 2019 was $490 billion
[Centers for Disease Control and Prevention, 2022]
Phase One
Relationship
Step 1: Facility Review
- Gaining a complete understanding of the facility, the processes & strategies, and potential risks.
Step 2: Setting the Standard
- Establishing a best practice strategy for ligature resistant products within the facility.
Step 3: Sampling & Approvals
- Providing full-size product samples for the facility and key stakeholders to review, including nursing staff and patient safety managers.
Phase Two
Project Lifecycle
Step 4: Project Specification
- Kingsway’s in-house Project and Design teams come together to define the scope of the project.
Step 5: Manufacture & Delivery
- The specified products are put into production in our Michigan based production facility and delivered to site.
Phase Three
Aftercare
Step 6: Annual Safety Check
- Ensuring the ligature resistant strategy is continuing to deliver life-saving benefits.
Step 7: Compliance Review
- Supporting the facility in keeping their systems up to date and ensuring continued compliance.

Want to begin your De-Risk Process?
Request a Facility Review to receive a no-obligation, comprehensive report identifying areas of ligature concern and detailing best practice recommendations to minimize risk within your behavioural health care environment.
- No-obligation Facility Review
- Comprehensive written report
- Identify areas of ligation concern and patient & staff safety risks
- Expert recommendations based on over 10,000 projects delivered worldwide
Simply request a Facility Review below and we’ll be in touch or contact us for more information.
